Predicting Entropic Effects of Water Mixing with Ionic Liquids
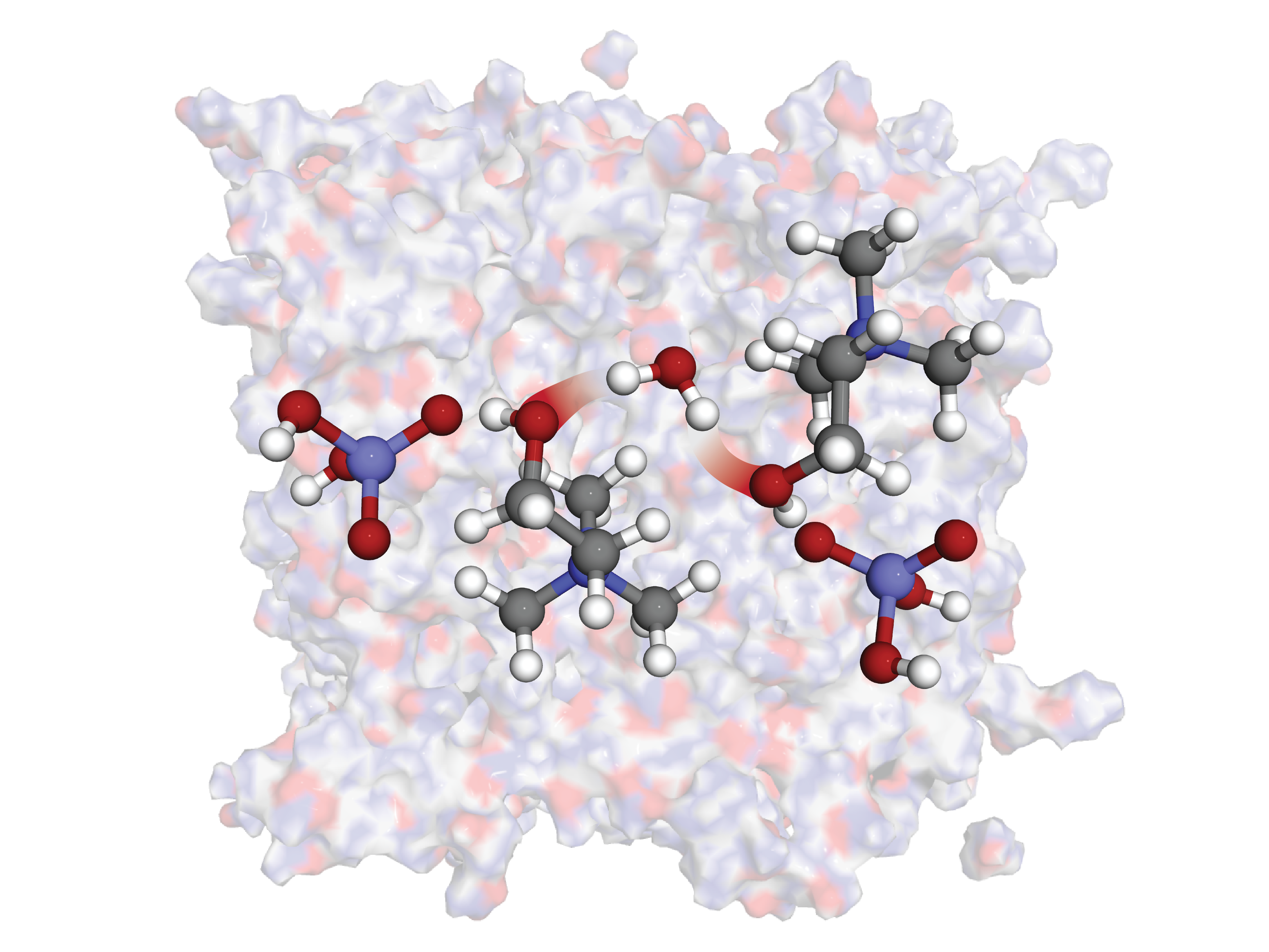
Study into the interactions of water with various hydrogen-bonding ionic liquids. We show that water incorporates itself into choline-based ionic liquids with minimal adverse effects on the neat ionic structure, resulting in a lower entropic penalty when compared to imidazolium-based ILs. Electronic properties were studied along with effects on IR spectra from a theoretical perspective.